Pioneers of Regeneration

In the world of regenerative medicine, the realm of science fiction is infiltrating the domain of real science. At Texas Heart Institute (THI), cadaverous pig hearts, stripped of their cellular makeup, exude stark, ethereal beauty while cardiac tissue stem cells pulse rhythmically in a dish. Across the street at the Houston Methodist DeBakey Heart & Vascular Center, a metamorphosis is underway as researchers work to transform human scar cells into blood vessel cells. A few blocks away at Baylor College of Medicine, scientists strive to harness the natural capabilities of our immune system to treat cancer. At The University of Texas Health Science Center at Houston (UTHealth) Medical School, stem cells are being utilized to repair traumatic brain injury in children. As science delves further into uncharted territory, Rice University’s Baker Institute for Public Policy examines ethical and policy issues stemming from these new developments. It’s a brave new world.
“Regenerative medicine and stem cells are a very large part of the future of medicine—all kinds of medicine, not just the heart,” noted James T. Willerson, M.D., president of Texas Heart Institute. “The brain, the kidneys, the eyes, the ears…everything. We’re all a product of stem cells.”
Possessing the remarkable ability to differentiate into a multitude of specialized cell types, as well as self-perpetuate through mitosis, stem cells represent a goldmine of biomedical possibilities. Willerson began probing those possibilities as early as the 1990s, when he was conducting stem cell work in experimental animal models. It wasn’t until 2000 that things really gained momentum. Alongside Emerson C. Perin, M.D., Ph.D., medical director of Texas Heart Institute’s Stem Cell Center, Willerson was part of an effort to perform the first ever injection of stem cells into the heart for patients with heart failure in Rio de Janeiro, Brazil.
“That’s how it all got started,” reflected Perin, a native Brazilian, who realized that navigating regulatory barriers might be easier in his home country. “At the time, we used adult bone marrow cells, which weren’t too specialized. Since then, we’ve really progressed along in trying to improve our process, every step of the way. We published that first experience in 2003, which led to us bringing the process here to Houston—we became very well known as pioneers in treating heart failure with stem cells.
“Since we had done that successfully, we decided not to limit ourselves to bone marrow cells,” he added. “We led several different trials, the first in humans, where we’ve put different types of stem cells into the heart. There’s also a population of cells called mesenchymal stem cells, which have an enormous therapeutic capacity for tissue repair. All of these other therapies were autologous, using the patient’s own cells, and for the first time these were allogeneic cells that came from one young donor. These different trials are all focused on treating heart failure, which is really our passion to do something about.”
In addition to treating 45 people with the mesenchymal cells from a single donor, Robert J. Schwartz, Ph.D., director of Stem Cell Engineering at Texas Heart Institute, and his team at Texas Heart Institute have also successfully generated cardiac stem cells by taking a biopsy from a human hand and converting them into heart muscle cells using genetic factors—a variation on the concept of induced pluripotent stem cells (iPSCs).
In 2006, Shinya Yamanaka, M.D., Ph.D., a Japanese Nobel Prize-winning stem cell researcher, was able to generate iPSCs through the activation of just four genes, resulting in a cell that possesses the same characteristics as an embryonic stem cell. “Those iPSCs are what you were when you were just a twinkle in your mother’s eye,” explained John P. Cooke, M.D., Ph.D., director of the Center for Cardiovascular Regeneration at Houston Methodist DeBakey Heart & Vascular Center. “They’re basically like an embryonic stem cell, but they’re your embryonic stem cell—they can be differentiated into any tissue of the body, from the brain to the pancreas. It’s difficult to study the brain cells of an individual with brain disease, for example, but you can take their skin, make iPSCs and then differentiate them into brain cells. Then you can study the disease in a dish.”
“When you begin to understand that genetic signaling to create a heart muscle cell, then you can take a biopsy from another place and turn them into contracting heart cells,” added Willerson. “Our goal is the regeneration of the whole heart, or at least enough of it so that a very weak heart would be made anew.”
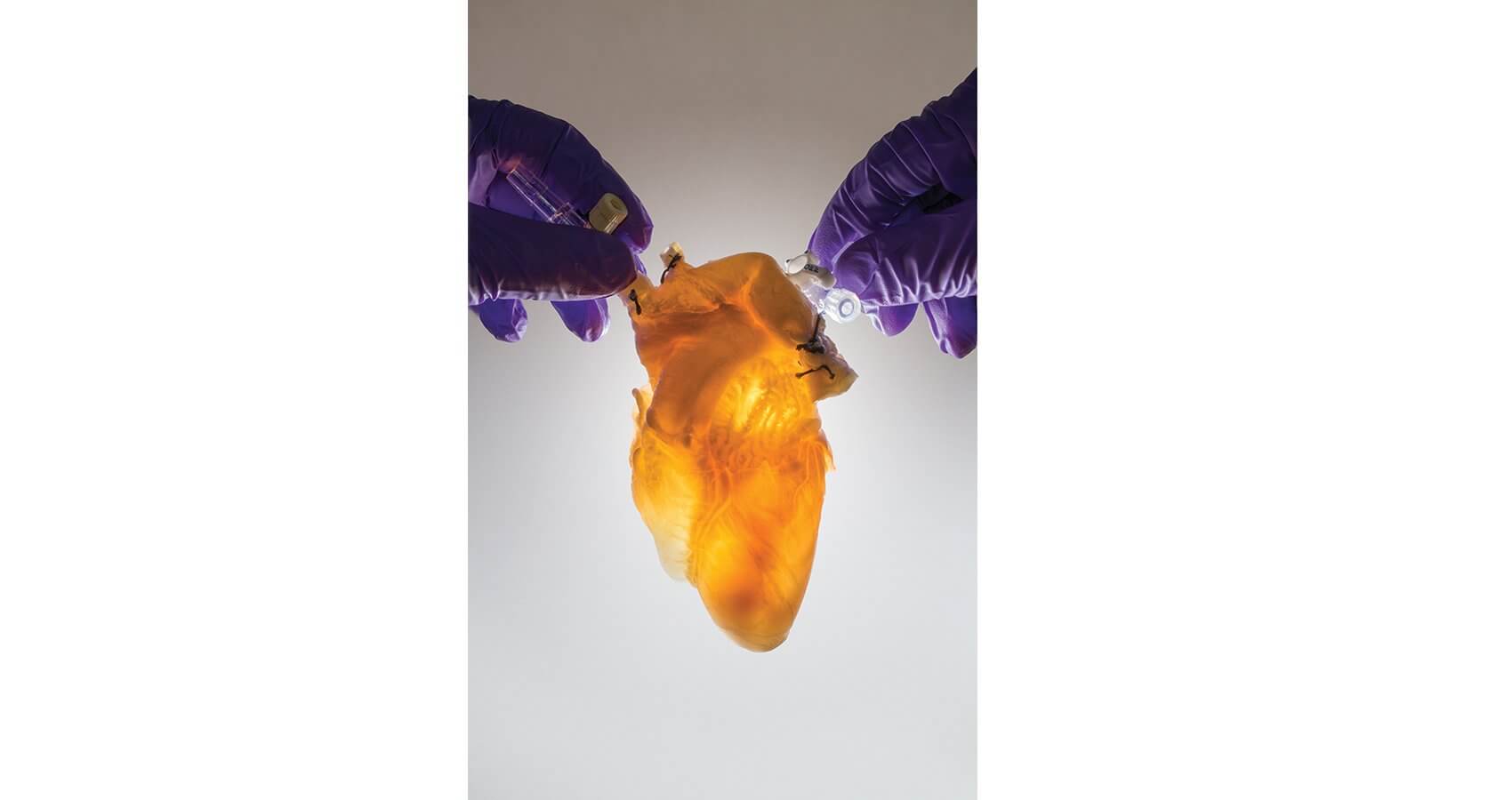
In the lab of Doris A. Taylor, Ph.D., director of Regenerative Medicine Research at Texas Heart Institute, where decellularized organs suspended in jars and submerged in Pyrex dishes line the shelves and adorn countertops, concrete steps are being taken along a similar trajectory. Her goal? Building a human heart.
“I take a very simplistic view of the world,” admitted Taylor. “Don’t imagine what you can’t do— just assume you can do it and give it a try and see what happens. A number of years ago, when we said we wanted to build complex, solid organs and tissue, we admitted that we weren’t tissue engineers, but still challenged ourselves to determine what the process entails.
“We had been doing cell therapy for years and knew that it obviously requires cells,” she said. “There are a number of sources for cells, from bone marrow to tissue and even generating iPSCs. It takes more than a few cells, though—if you’re going to build an organ, it’s going to take hundreds of billions of cells. More than that, we need a place to put those cells.”
Rather than pursuing labor-intensive methods to create a bio printed or mechanical scaffold, Taylor chose to piggyback on preexisting structures in nature. Along with her colleagues, she was able to take a human-sized pig organ—a heart, initially—and remove all the cells, leaving a completely acellular construct. The technique has also been applied to a liver, kidney, gallbladder and pancreas. Working with colleagues around the world, Taylor has just begun to recellularize those constructs.
“Decellularization is very simple,” she explained. “It’s essentially just washing out the cells. You have something that goes from a red, muscularized organ to an acellularized construct over a relatively short period of time, while leaving the extracellular matrix composition intact—it literally looks like you took a tube of toothpaste and squeezed. What’s left is the structural composition.
“We have the ability to recapitulate a whole heart,” she added. “We determined that we could take one of these decellularized hearts and simply put cells back into the vascular tree to recellularize the whole network.” Taylor is driven to ultimately reseed hearts using cells from a given individual to manufacture an available, tailor-made organ for transplantation.
“One of the critical unmet needs in pediatric care is the lack of a reparative therapy for traumatic brain injury—that’s really where I first became engaged in the use of stem cell based therapies.” — Charles Cox Jr., M.D., Professor of Pediatric Surgery and Director of the Pediatric Surgical Translational Laboratories and Pediatric Program in Regenerative Medicine at UTHealth Medical School
Researchers at Texas Heart Institute aren’t the only ones vying for new ways to regenerate damaged tissue. At the Houston Methodist DeBakey Heart & Vascular Center, cardiovascular scientists are exploring a medical approach for transforming human heart cells into blood vessel cells.
“This approach, known as transdifferentiation, actually avoids cell-based therapy entirely,” said Cooke. “In a situation where you have an injury, a small molecule cocktail could potentially transdifferentiate some of those cardiac fibroblasts—a cell type that causes scarring and is plentiful throughout the body—into endothelium, an entirely different type of adult cell that forms the lining of new blood vessels.”
Chronicled in a recent issue of Circulation, this method provides proof-of-concept for a small molecule therapy that could one day be used to improve healing of cardiovascular damage or other injuries.
“What we’ve discovered is something that lower vertebrates use to regenerate tissues, where their cells become more plastic in their phenotypes,” said Cooke. “It’s a very primordial response—once a cell is confronted by damage or a pathogen, it has to react and change its phenotype to become more fluid. Now that we understand this phenomenon, we’re looking at the potential in manipulating that, therapeutically.”
Leveraging the plasticity of certain types of cells is also within the purview of immunologists at Baylor College of Medicine, who are invested in harnessing the immune system to treat cancer.
“The crux of our work has less to do with regenerative medicine and more to do with the plasticity of blood stem cells and how they can be harvested in treating disease,” explained William K. Decker, Ph.D., assistant professor of immunology at Baylor. “Immune cells are even more plastic than people realize. When they’re stimulated in different ways, they really have this tremendous differentiative potential and can turn into highly specialized subtypes for addressing various kinds of immunological conditions.
“We’re really interested in generating these highly specific, cancer-fighting immune cells from regular immune cells,” he continued. “We study the signals required to turn ordinary cells into these super, cancer-fighting immune cells, and we’re interested in a broad variety of cancers.”
A process that uses the body’s own immune system to treat cancer instead of relying on external treatments like chemotherapy, cancer immunotherapy may have the ability to stave off metastasis, while also offering a reduced side effect profile. Decker and his colleagues hope to train the immune system to recognize cancer, preventing it from returning after it has been eradicated.
“People will still need chemotherapy and radiotherapy, but hopefully the doses can be smaller and the side effects more manageable,” he said. “We envision this working in a way that prevents subsequent metastasis, so that it might have a real impact on both relative and absolute longevity.”
At UTHealth Medical School, stem cell research is being used as an innovative approach to treat traumatic brain injury in children.
“One of the critical unmet needs in pediatric care is the lack of a reparative therapy for traumatic brain injury—that’s really where I first became engaged in the use of stem cell based therapies,” reflected Charles Cox, Jr., M.D., professor of pediatric surgery and director of the Pediatric Surgical Translational Laboratories and Pediatric Program in Regenerative Medicine at UTHealth Medical School. “The development of the Pediatric Program in Regenerative Medicine came out of that unmet need.”
Cox, who also directs the Pediatric Trauma Program at Children’s Memorial Hermann Hospital, aims to address problems that originate with a severe traumatic injury—the ones resulting in coma and placing the young trauma victims at risk for permanent disability. To mitigate that risk, he recently completed the first acute autologous cell therapy treatment for traumatic brain injury.
The applications of stem cell therapy in neurological injuries or disorders are extensive—Sean Savitz, M.D., professor of neurology at UTHealth Medical School, and his research teams have realized that adult derived stem cells from a patient’s own bone marrow have the capacity to enhance recovery following a stroke.
“We started looking at mechanisms for that reparative potential and found that the cells are potentially releasing a number of biological factors that help
the brain heal itself,” Savitz explained. “It became a very different approach to how we think stem cells function.” In July 2011, the first patient in Texas was enrolled in the country’s first double-blind clinical trial studying the safety and efficacy of a stem cell therapy technique that can be given up to 19 days after an ischemic stroke. Savitz believes that expanding the time window for administering stem cells increases the number of patients who might be helped.
“I see really high potential for developing cell therapies for a range of neurological disorders,” he added. “I think that’s partly a goal here, thinking about which disorders might be amenable in using cell therapies and doing properly designed, rigorous clinical trials to assess their safety and effectiveness. I don’t think the way that cell therapies are being applied is going to completely cure neurological disease, but I think some of them have the potential to slow down the course of neurodegenerative disorders.”
Rebuffing misconceptions is an essential aspect of any burgeoning discipline. At Rice University’s Baker Institute for Public Policy, Kirstin R.W. Matthews, Ph.D., the fellow in science and technology policy, wanted to explore those dynamics.
“My research focuses on ethical and policy issues related to biomedical research and development,” she said. “Specifically, I am looking at intellectual property rights for biotechnology, including genetics and stem cell related patents. Initially, when all of these decisions were being made, it seemed like they were occasionally being politically manipulated—often due to a lack of understanding of the science behind them.”
In a recent paper that she co-authored with Maude Rowland Cuchiara, Ph.D., the Baker Institute scholar for science and technology policy, Matthews examined how some National Football League (NFL) players have been seeking out unproven stem cell therapies to help accelerate recoveries from injuries. While most players seem to receive treatment within the United States, several have traveled abroad for therapies unavailable domestically and may be unaware of the risks.
“With the rise of new and unproven stem cell treatments, the NFL faces a daunting task of trying to better understand and regulate the use of these therapies in order to protect the health of its players,” said Matthews. “The online data on NFL players and the clinics where they obtained treatment suggests that players may be unaware of the risks they are taking. Furthermore, players who are official spokespersons for these clinics could influence others to view the therapies as safe and effective despite the lack of scientific research to support these claims.”
Her paper was published in the 2014 World Stem Cell Report—a special supplement to the journal Stem Cells and Development. This past year, the Summit was held in San Antonio, Texas. Matthews, Cox, Decker, Cooke, Taylor, and Willerson all gave presentations illustrating highlights of their work in regenerative medicine.
“There are multiple ways that these advances in regenerative medicine can change the landscape of health sciences,” affirmed Taylor. “I see regenerative medicine providing hope for people who haven’t had hope for years, as well as alternatives—not just to treat the symptoms but to treat the underlying disease. That way, we can actually move people backwards on the disease continuum rather than simply palliating their symptoms.
“I truly hope participants at the World Stem Cell Summit walked away realizing that Texas is a place where, if you can imagine it, you can do it,” she added. “It’s not crazy to have a Stem Cell Summit in the middle of Texas. It’s actually very progressive, because we have some of the best thinkers in the world here.”
AN INTRODUCTION TO STEM CELLS
What are stem cells?
Stem cells have the remarkable potential to develop into many different cell types in the body during early life and growth. In addition, in many tissues they serve as a sort of internal repair system, dividing essentially without limit to replenish other cells as long as the person or animal is still alive. When a stem cell divides, each new cell has the potential either to remain a stem cell or become another type of cell with a more specialized function, such as a muscle cell or red blood cell.
What makes stem cells unique?
Stem cells differ from other kinds of cells in the body. All stem cells, regardless of their source, have three general properties: they are capable of dividing and renewing themselves for long periods; they are unspecialized; and they can give rise to specialized cell types.
What are embryonic stem cells?
Embryonic stem cells, as their name suggests, are derived from embryos. Most embryonic stem cells are derived from embryos that develop from eggs that have been fertilized in vitro—in an in vitro fertilization clinic—and then donated for research purposes with informed consent of the donors. They are not derived from eggs fertilized in a woman’s body.
What are adult stem cells?
An adult stem cell is thought to be an undifferentiated cell, found among differentiated cells in a tissue or organ. The adult stem cell can renew itself and can differentiate to yield some or all of the major specialized cell types of the tissue or organ. The primary roles of adult stem cells in a living organism are to maintain and repair the tissue in which they are found.
Adult stem cells have been identified in many organs and tissues, including brain, bone marrow, peripheral blood, blood vessels, skeletal muscle, skin, teeth, heart, gut, liver, ovarian epithelium, and testis. They are thought to reside in a specific area of each tissue (called a “stem cell niche”). Stem cells may remain quiescent (non-dividing) for long periods of time until they are activated by a normal need for more cells to maintain tissues, or by disease or tissue injury.
What are the similarities and differences between embryonic and adult stem cells?
Human embryonic and adult stem cells each have advantages and disadvantages regarding potential use for cell-based regenerative therapies. One major difference between adult and embryonic stem cells is their different abilities in the number and type of differentiated cell types they can become. Embryonic stem cells can become all cell types of the body because they are pluripotent, while adult stem cells are thought to be limited to differentiating into different cell types of their tissue of origin.
Adult stem cells, and tissues derived from them, are currently believed less likely to initiate rejection after transplantation. This is because a patient’s own cells could be expanded in culture, coaxed into assuming a specific cell type (differentiation), and then reintroduced into the patient. The use of adult stem cells and tissues derived from the patient’s own adult stem cells would mean that the cells are unlikely to be rejected by the immune system. This represents a significant advantage, as immune rejection can be circumvented only by continuous administration of immunosuppressive drugs, and the drugs themselves may cause deleterious side effects.
What are induced pluripotent stem cells?
Induced pluripotent stem cells (iPSCs) are adult cells that have been genetically reprogrammed to an embryonic stem cell-like state by being forced to express genes and factors important for maintaining the defining properties of embryonic stem cells. Although these cells meet the defining criteria for pluripotent stem cells, it is not known if iPSCs and embryonic stem cells differ in clinically significant ways. Mouse iPSCs were first reported in 2006, and human iPSCs were first reported in late 2007.
iPSCs are already useful tools for drug development and modeling of diseases, and scientists hope to use them in transplantation medicine. This breakthrough discovery has created a powerful new way to “de-differentiate” cells whose developmental fates had been previously assumed to be determined. In addition, tissues derived from iPSCs will be a nearly identical match to the cell donor and thus probably avoid rejection by the immune system. The iPSC strategy creates pluripotent stem cells that, together with studies of other types of pluripotent stem cells, will help researchers learn how to reprogram cells to repair damaged tissues in the human body.
What are the potential uses of human stem cells?
There are many ways in which human stem cells can be used in research and the clinic. Studies of human embryonic stem cells will yieldinformationaboutthecomplexeventsthatoccurduringhuman development. A primary goal of this work is to identify how undifferentiated stem cells become the differentiated cells that form the tissues and organs.
Human stem cells are currently being used to test new drugs. New medications are tested for safety on differentiated cells generated from human pluripotent cell lines. Other kinds of cell lines have a long history of being used in this way. Cancer cell lines, for example, are used to screen potential anti-tumor drugs.
Perhaps the most important potential application of human stem cells is the generation of cells and tissues that could be used for cell-based therapies. Today, donated organs and tissues are often used to replace ailing or destroyed tissue, but the need for transplantable tissues and organs far outweighs the available supply. Stem cells, directed to differentiate into specific cell types, offer the possibility of a renewable source of replacement cells and tissues to treat a wide range of diseases.